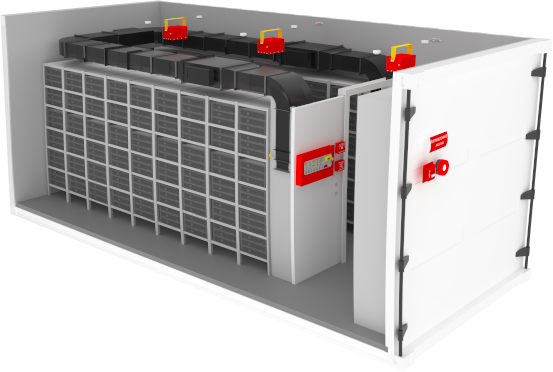
In terms of operational performance, our technology has demonstrated impressive levels of efficiency and effectiveness. We have accumulated over 7 years of data from more than 100 accredited laboratory tests and prominent Li-Ion battery manufacturers which confirm our technology's capability to suppress Li-Ion battery fires. Our comprehensive and intricate analysis was made possible by a purpose-built spherical test chamber designed specifically to assess the multifaceted fire, explosion, and off-gassing behavior of Li-Ion batteries.
As a result of our meticulous approach, we have successfully established our technology's proven track record of delivering consistent and reliable results. Our results are a testament to the exceptional engineering and innovation put into our technology, which has enabled it to stand out as a leader in the industry. We are confident that our technology's efficacy will enable us to fulfill the needs and expectations of our customers and partners while maintaining our position as a top-tier business in the field of Li-Ion battery safety.